Drug-Induced Thrombocytopenia
Drug-induced thrombocytopenia is just what it sounds like: low platelet counts due to a side effect of a medication. Join us in learning about some key culprit drug and how to manage these cases!

Ideally, drug are supposed to treat and/or cure our patients. Unfortunately, medication use does not come risk-free. There are many drugs that can reduce the platelet count as a side-effect. This is called drug-induced thrombocytopenia.
You may be wondering: "if we know a drug is causing the platelet count to drop, why don't we stop the drug?"
If only it were that simple all of the time!
Here enters the role of the clinical pharmacist. The clinical pharmacist can help identify culprit medications, recognize drug-induced side effects, and help the team manage the delicate balance of treating the patient appropriately while minimizing adverse drug effects.
In this topic discussion, we will be discussing four main points:
- General medications that cause drug-induced thrombocytopenia
- Heparin- induced thrombocytopenia
- Linezolid-induced thrombocytopenia
- Trimethoprim-sulfamethoxazole-induced thrombocytopenia
Special thanks to a previous guest author, Brett White, PharmD, for the majority of this information today!
Introduction
Thrombocytopenia – Platelet count < 150 x 109/L
- Platelet count, a component of complete blood count (CBC), is generally assessed daily in hospitalized patients.
Pathogenesis
- Decreased platelet production – Cirrhosis, malignancy, alkylating agents
- Increased platelet destruction – Drug-induced, thrombotic thrombocytopenic purpura (TTP), disseminated intravascular coagulation (DIC), hemolytic uremic syndrome (HUS)
- Increased splenic sequestration – Most commonly in sickle cell patients
- Dilution – Aggressive fluid resuscitation, continuous maintenance fluids
Clinical Concern
- Majority of cases – bleeding
- Heparin-induced thrombocytopenia (HIT) – thrombus formation
Epidemiology
- Drug-induced immune thrombocytopenia (DITP) has been reported to account for 20% to 25% of all drug-related blood dyscrasias (an abnormal state of the body).
- Estimated incidence – 10 cases per million population per year.
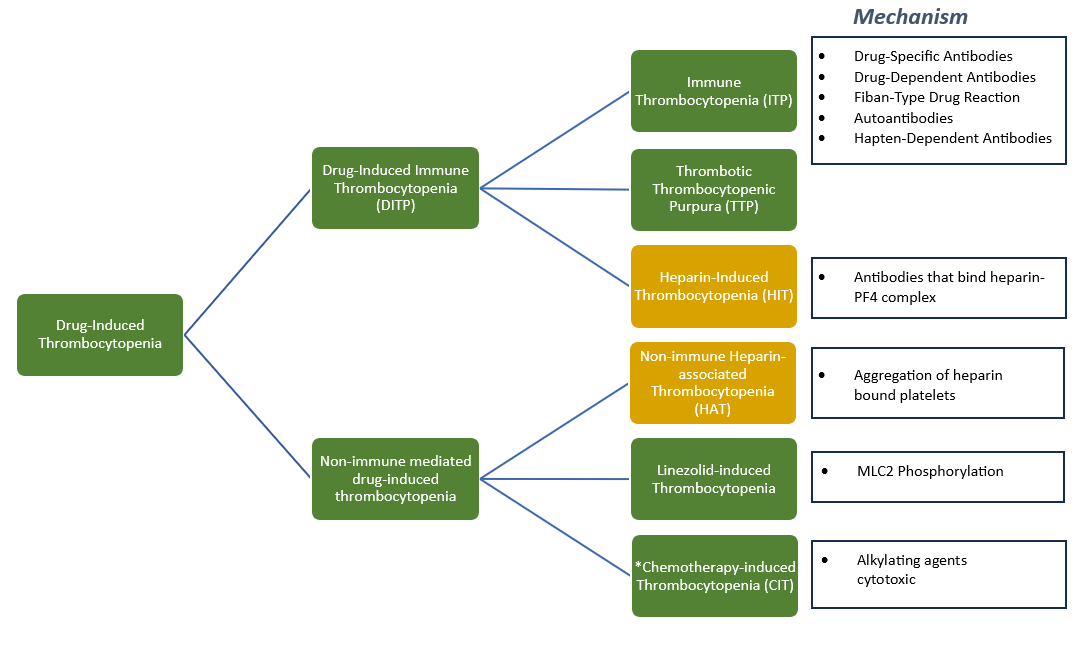
So... What are the culprit drugs?
Offending Agent List*
Abciximab
Acetaminophen
Amiodarone
Ampicillin
Carbamazepine
Cephalosporins
Eptifibatide
Ethambutol
Ganciclovir**
Haloperidol
Ibuprofen
Irinotecan
Naproxen
Oxaliplatin
Phenytoin
Piperacillin
Quinidine
Quinine
Ranitidine
Rifampin
Simvastatin
Tirofiban
Trimethoprim-Sulfamethoxazole
Valganciclovir**
Valproic Acid
Vancomycin
*Not all inclusive; adapted from multiple references in which evaluation included clinical criteria; reports of drug-dependent, platelet-specific antibodies; and reports from Adverse Events Reporting System (AERS)1,2,3,4
**Ganciclovir and Valganciclovir are not included in references 1-4, however, I have had real-life experience with platelet counts (in additional to white blood cell counts) dropping in transplant patients on these agents. Despite the poor cell counts, the medication was critical at the time, and stopping/limiting the use of other agents was preferred to allow ganciclovir/valganciclovir to stay on board.
What about Heparin-Induced Thrombocytopenia (HIT)?
- Incidence: 10-30%
- Onset < 5 days of heparin initiation, transient
- Platelet count nadir: Rarely < 100 x 109/L
- Mild platelet aggregation
- Management: observe, counts recover to baseline without holding heparin products
- 1-5% patients receiving IV treatment doses of heparin
- Bovine > porcine (incidence)
- Therapy duration dependent (≥ 6 days) and frequency of exposure dependent
- Surgical patients (orthopedic, trauma), heart transplant at highest risk
- Antibodies generated in response to epitope(s) of heparin – PF4 complex
HIT Clinical Presentation
- 50% platelet count reduction or < 150 x 109/L warrants work up.
- Onset 5-14 days of heparin initiation unless heparin exposure within past 100 days.
- Greatest concern is clotting.
- In setting of serologically confirmed HIT, associated thrombosis has been identified as late as 5-19 days after heparin product discontinuation.
- Thrombosis can occur at any point in time of platelet decline, even before beginning of downtrend.
- Atypical presentation – skin necrosis with venous gangrene, disseminated intravascular coagulation (DIC), and anaphylactic reactions with bolus.
Clinical Pearls
- Incidence of HIT is 10-fold higher with UFH administration relative to LMWH
- A few cases of HIT have been reported with Fondaparinux administration, but this is extraordinarily rare (used in management of HIT).
- Warfarin should NEVER be used in the initial management of HIT as reduction in protein C and S potentiates hypercoagulability.
- However, warfarin a reasonable agent for completion of 4 week anticoagulation therapy after platelet counts recover > 150 x 109/L.
- Overlap of 4-5 days of warfarin and non-heparin agent used for initial management required when initiating warfarin.

4-T Scoring – Check out this online calculator!
|
|
2 point |
1 points |
0 points |
|
Thrombocytopenia |
Platelet
count reduction > 50%
AND nadir ≥ 20 x 109/L AND no surgery in previous 3 days |
Platelet count reduction of 30-50% or platelet nadir 10-19 x 109/L *Platelet count reduction > 50% BUT surgery
within previous 3 days |
< 30% platelet count reduction or platelet nadir <
10 x 109/L |
|
Timing of platelet count fall |
Clear onset
days 5-10 or platelet reduction within 24 hours (prior heparin exposure
within 30 days) |
Consistent with days 5-10 fall, but not clear (eg
missing platelet counts); onset after day 10 or fall ≤ 1 day (prior heparin
exposure 30-100 days ago) |
Onset ≤ 4 days without recent exposures |
|
Thrombosis or other sequelae |
New
thrombosis (confirmed); skin necrosis; acute systemic reaction post IV UFH
bolus |
Progressive or recurrent thrombosis; non-necrotizing
(erythematous) skin lesions; suspicion of thrombosis (not proven) |
None |
|
Other causes of thrombosis |
None apparent |
Possible |
Definite |
Assessment of 4-T scoring
>> Score ≤ 3 points – Low probability of HIT
>> Score of 4-5 – Intermediate probability of HIT
>> Score ≥ 6 points – High probability of HIT
- Scoring consistent with intermediate and high probability of HIT warrants further workup with immunoassay
- Positive immunoassay + High probability of HIT = confirmatory
- Positive immunoassay + Intermediate probability of HIT = 40-60% likelihood of HIT
- Presence of high IgG OD/titer and/or platelet activating antibodies increases HIT likelihood – 65%
Management of confirmed or high suspicion of HIT
Selection of one of the agents from the following classes of anticoagulants:
- Direct Thrombin Inhibitors (DTIs) – Argatroban, Lepirudin, Bivalirudin
- Factor Xa Inhibitors – Fondaparinux
- Heparinoids – Danaparoid
TIP: Check with what is on formulary at your institution.
ACCP Guidelines recommend a duration of 4 weeks of anticoagulation therapy for isolated cases of HIT, but 3 months for HIT complicated by thrombosis.
DOACs demonstrated to have no cross-reactivity with HIT antibodies, study published in October 2022 demonstrating higher rate of pulmonary embolism, but lower mortality relative to DTI’s.

Linezolid-Induced Thrombocytopenia
Risk Factors
- Use ≥ 14 days
- CrCl < 60 mL/min
- Hemodialysis
- Baseline platelet count ≤ 200 x 109/L
- Higher daily weight-based dose (per kg)
- Cmin > 2 mg/L when given for an extended duration
- Preexisting myelosuppression
- Concurrent medications that cause bone marrow suppression
- Chronic infection (previous or concurrent antibiotic therapy)
Presentation
Patients generally begin dropping their counts between days 10-14 of therapy in the setting of prolonged therapy (> 14 days). Concern for bleeding.
3% incidence of thrombocytopenia in adults treated up to 28 days reported in initial phase III studies that led to linezolid’s approval by the FDA.
However, Crass et al9 demonstrated a thrombocytopenia (PLT < 112.5 x 109/L) incidence of 27% and severe thrombocytopenia (PLT < 75 x 109/L) incidence of 47% in a population size of 341 patients with a greater frequency of both thrombocytopenic states among patients with renal impairment (CrCl < 60 mL/min). Unclear how long these patients were treated with linezolid.
Mechanism
Tajima et al10 were able to demonstrate in vitro increased phosphorylation of myosin light chain 2 (MLC2) which mediates platelet release from megakaryocytes in the bone marrow (Phosphorylation attenuates signaling capability of MLC2).
Management
Discontinue linezolid, counts will generally recover completely within 10-14 days.
Trimethoprim-Sulfamethoxazole-Induced Thrombocytopenia
Presentation
Generally, onset ranges from 5 days to 6 weeks, but can occur faster depending on previous sulfonamide exposure.
Mechanism
Non dose-related, immune-mediated, case reports have demonstrated presence of an anti-platelet antibody requiring presence of sulfamethoxazole to be synthesized.
Drug-dependent antibodies – Pre-existing antibodies that have a weak affinity to epitopes of GPIIb/IIIa or Ib/V/IX complexes (expressed on surface of platelets). Introduction of offending agent binds these glycoproteins, confers a conformational change, and subsequently increases the affinity of pre-existing antibodies to their specific epitope on surface of platelets.
Management
Discontinue offending agent, platelet counts generally recover > 150 x 109/L in 7 days.
We hope you enjoyed today's quick topic discussion! Be on the lookout for these potential culprit agents, and keep a keen eye on those platelets!
Additional references not linked throughout the article:
- Curtis BR. 2014. Drug-induced immune thrombocytopenia: incidence, clinical features, laboratory testing, and pathogenic mechanisms. Immunohematology 30(2): 55–65.
- Aster RH, Curtis BR, McFarland JG, et al. 2009. Drug‐induced immune thrombocytopenia: pathogenesis, diagnosis, and management. Journal of Thrombosis and Haemostasis 7(6): 911–8.
- Reese, J.A., Nguyen, L.P., Buchanan, G.R., Curtis, B.R., Terrell, D.R., Vesely, S.K. and George, J.N. (2013), Drug-induced thrombocytopenia in children. Pediatr Blood Cancer, 60: 1975-1981.
- George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009:153-8.
- Kam T, Alexander M. Drug-induced immune thrombocytopenia. J Pharm Pract. 2014;27(5):430-439.
- Lee GM, Arepally GM. Heparin-induced thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2013;2013:668-674.
- Zyvox (linezolid) [prescribing information]. New York, NY: Pharmacia and Upjohn; August 2022.
- Gerson SL, Kaplan SL, Bruss JB, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46(8):2723-2726.
- Crass RL, Cojutti PG, Pai MP, Pea F. Reappraisal of Linezolid Dosing in Renal Impairment To Improve Safety. Antimicrob Agents Chemother. 2019;63(8):e00605-19. Published 2019 Jul 25.
- Tajima M, Kato Y, Matsumoto J, et al. Linezolid-Induced Thrombocytopenia Is Caused by Suppression of Platelet Production via Phosphorylation of Myosin Light Chain 2. Biol Pharm Bull. 2016;39(11):1846-1851.
- Barr AL, Whineray M. Immune thrombocytopenia induced by cotrimoxazole. Aust N Z J Med. 1980;10(1):54-55
- Kiefel V, Santoso S, Schmidt S, Salama A, Mueller-Eckhardt C. Metabolite-specific (IgG) and drug-specific antibodies (IgG, IgM) in two cases of trimethoprim-sulfamethoxazole-induced immune thrombocytopenia. Transfusion. 1987;27(3):262-265.
- Myers MW, Jick H. Hospitalization for serious blood and skin disorders following co-trimoxazole. Br J Clin Pharmacol. 1997;43(6):649-651.
*Information presented on RxTeach does not represent the opinion of any specific company, organization, or team other than the authors themselves. No patient-provider relationship is created.

