Opportunistic Infections: Pneumocystis jirovecii Pneumonia (PJP)
Our first clinical pharmacy article of the year is a high-level overview of pneumocystis jiroveci pneumonia, or PJP (aka PCP). Stay tuned for future opportunistic infection posts!
What are opportunistic infections?
- Opportunistic infections (OIs) are illnesses that are more frequent and more severe in immunocompromised individuals due to immunosuppression.
- Immunocompromised means that your immune system is weakened either by disease or by a medication
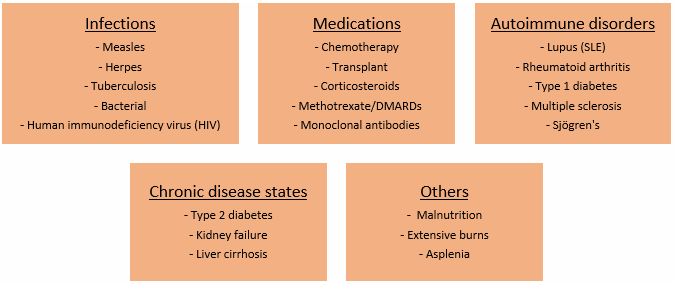
Several infections, disease states, medications, and disorders can compromise someone's immune system (Figure 1).
Although there are many OIs out there, we will focus on Pneumocystis jirovecii pneumonia (PJP) for today!
Types of prophylaxis
- Primary: prophylaxis before the disease has occurred or the microorganism has entered the body
- Secondary: prevents the disease after having been infected or treated
- In other words...
- Primary = preventing the first episode
- Secondary = preventing recurrent episodes
Pneumocystis jirovecii Pneumonia (PJP)
Incidence
PJP is caused by the fungus Pneumocystis jirovecii (used to be Pneumocystis carinii but this is no longer used after a taxonomy reclassification when it became clear jirovecii infects humans and carinii infects rats)
- In the HIV population, PJP occurred in up to 80% of patients with AIDS prior to ART and PJP prophylaxis being a regular in clinical practice guidelines
- Now cases are less than 1 per 100 person years. PJP mainly occurs in patients who are unaware they have HIV
- The risk of PJP is 90% when the CD4 count falls below 200 cells/mm3
- The rate of PJP in solid organ transplant recipients is estimated at 0.3%-2.6% based on data from 2015
- In cancer patients with solid tumors, the rate is as low as 0.05%
Transmission
- Airborne route
- Patients can inhale a new virus or reactivate a latent infection
Presentation
- Subacute, nonproductive cough, progressive dyspnea, and fever
- Pulmonary physical exam (PE) is usually normal except for in advanced disease, rales can be heard
Diagnosis
- Non-definitive tests
- Chest radiograph
- High-res chest CT
- Exercise Pulse Ox
- Lab studies:
- LDH >500 mg/dL
- 1,3 beta-D-glucan >80 pg/mL (major component of cell wall)
- Definitive tests
- Induced sputum – LOW sensitivity and should not be submitted for diagnosis
- Bronchoscopy (BAL) – HIGH sensitivity (95%)
- Transbronchial or open-lung biopsy: rarely required due to BAL results
- Detection of PJP organisms in sample: direct immunofluorescent stain procedure of choice. PCR is sensitive but does not distinguish between colonization and infection
Primary prophylaxis
- HIV patients
- CD4 less than 200 cells/mm3
- CD4 percent less than 14% of the total lymphocyte count
- CD4 200-250 cells/mm3 if antiretroviral therapy (ART) must be delayed and CD4 monitoring (every 3 months) is not possible
- Solid organ transplant patients
- 6-12 months post-transplant depending on institutional protocol
- Cancer patients
- Largely dependent on the type of cancer being treated, the chemotherapy regimen, and the expected neutropenia
- Other autoimmune disorders/conditions that cause immunosuppression
- Some experts suggest for patients receiving corticosteroids at doses equivalent to at least 20 mg/day of prednisone for at least 4 weeks should receive PJP prophylaxis

Treatment
- Suspected PJP should be started on treatment if they have documented partial pressure of oxygen (PaO2) >70 mmHg and a calculated P(A-aO2) gradient less than 35 mmHg
- All durations are 21 days minimum
- Mild – PaO2 >70 mmHg and calculated gradient <35 mmHg
- Moderate – PaO2 <70 mmHg OR alveolar‐arterial O2 gradient >35 mmHg on room air
- Moderate-severe: use of corticosteroids improves survival. Must have the PaO2 <70 or gradient >35

- Update 1/9/2025: an alternative treatment regimen for mild-to-moderate disease includes primaquine + clindamycin
- PO primaquine 30 mg OD +
- Clindamycin: IV 600 mg q6h or 900 mg q8h – or – PO 450 mg q6h or 600 mg q8h
Discontinuing prophylaxis
It is important to discontinue prophylaxis as soon as it is safe to do so to lower pill burden, minimize the risk of toxicity, reduce any drug interactions, and prevent resistance
- HIV population
- CD4 >200 cells/mm3 for 3 months in response to ART (AI)
- Can consider when CD4 100-200 and HIV RNA levels remain below limit of detection for 3-6 months (BII)
- Solid organ transplant population
- After 6-12 months, per institutional protocol, unless as continued risk or severe immunosuppression
- Cancer patients
- When neutropenia resolves
- Other autoimmune disorders/conditions
- When successfully tapered off high-dose steroids
Secondary prophylaxis
- Start immediately upon completion of 21-day treatment and then d/c as above
Restarting prophylaxis
(specifically in the HIV population)
- CD4 count less than 100 regardless of HIV RNA levels or 100-200 if HIV RNA is detectable
Notable adverse effects of medication therapy
- Trimethoprim-sulfamethoxazole (Bactrim)
- Rash, fever, nausea, hyperkalemia, azotemia, leukopenia, thrombocytopenia, transient increase in liver enzymes
- Mean onset 10-14 days after starting
- Prior mild skin rash can reintroduce under supervision and gradually titrate up the dose
- AVOID rechallenging in patients with hepatitis, aseptic meningitis, SJS, or TENS
- Dapsone (Aczone)
- Cross reactivity with Bactrim allergy/intolerance. Both contain a sulfonamide moiety
- Can cause hemolytic anemia due to G6PD deficiency, methemoglobinemia
- Methemoglobinemia is a condition with life-threatening potential in which diminution of the oxygen-carrying capacity of circulating hemoglobin occurs due to conversion of some or all of the four iron species from the reduced ferrous [Fe2+] state to the oxidized ferric [Fe3+] state.), peripheral neuropathy, sulfone syndrome (fever, lymphadenopathy, rash, hepatitis, lymphocytosis)
- Inhaled pentamidine (Pentam)
- Cough and bronchospasm
- Atovaquone (Mepron)
- Bad taste, off-putting mustard color
References
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/whats-new
- Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents
- Iriart X, Bouar ML, Kamar N, Berry A. Pneumocystis Pneumonia in Solid-Organ Transplant Recipients. J Fungi (Basel). 2015 Sep 28;1(3):293-331. doi: 10.3390/jof1030293.
- Jeon CH, Kim SH, Kim S, Bae M, Lee SJ, Lim S. Pneumocystis jirovecii Pneumonia in Patients with Solid Malignancies: A Retrospective Study in Two Hospitals. Pathogens. 2022 Oct 11;11(10):1169. doi: 10.3390/pathogens11101169.
- Neumann S, Krause SW, Maschmeyer G, Schiel X, von Lilienfeld-Toal M; Infectious Diseases Working Party (AGIHO); German Society of Hematology and Oncology (DGHO). 2013 Apr;92(4):433-42. doi: 10.1007/s00277-013-1698-0. Epub 2013 Feb 15.
- Caplan AS, Mecoli CA, Micheletti RG. Prophylaxis Against Pneumocystis Pneumonia. JAMA. 2023;330(19):1908–1909. doi:10.1001/jama.2023.18862
- Ewald H, Raatz H, Boscacci R, Furrer H, Bucher HC, Briel M. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev. 2015 Apr 2;2015(4):CD006150. doi: 10.1002/14651858.CD006150.pub2.
- Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. Riverwoods, IL. http://online.lexi.com
- Ibrahim A, Chattaraj A, Iqbal Q, Anjum A, Rehman MEU, Aijaz Z, Nasir F, Ansar S, Zangeneh TT, Iftikhar A. Pneumocystis jiroveci Pneumonia: A Review of Management in Human Immunodeficiency Virus (HIV) and Non-HIV Immunocompromised Patients. Avicenna J Med. 2023 Mar 24;13(1):23-34. doi: 10.1055/s-0043-1764375.
*Information presented on RxTeach does not represent the opinion of any specific company, organization, or team other than the authors themselves. No patient-provider relationship is created.

